Will Indonesia-China vaccine cooperation affect Jakarta's South China Sea stance?

June 2020, three months after announcing its first two Covid-19 cases, Indonesia saw a continuous rise in the number of confirmed cases despite efforts to limit social and economic activities as well as citizen movement in many major cities. The country's economy was also heading to its sharpest downturn since the 1997/8 Asian financial crisis, and had contracted 5.32% in the second quarter of 2020.
Months after the outbreak, Indonesia explored vaccine cooperation with China, South Korea, the UAE and the UK. In October 2020, China started to offer vaccine assistance to Southeast Asian countries, with Foreign Minister Wang Yi conducting vaccine diplomacy by touring Southeast Asia in October 2020 and January 2021. This made Beijing's assistance in the region - in terms of coping with the pandemic - unrivalled by any other power. In July 2020, Xiao Yaqing, the head of the Bureau of Market Supervision and Control, during his visit to Sinopharm, urged researchers to consider the development of a Covid-19 vaccine as "an important political task".
He [President Joko Widodo] officially kicked off the vaccine rollout in the country by receiving the jab himself, becoming the first non-Chinese leader to be inoculated with the Chinese vaccine.
On 13 January 2021 after marathon diplomatic efforts and bureaucratic talks between Jakarta and Beijing, President Joko Widodo became the first Indonesian to receive a dose of Sinovac Biotech's CoronaVac. He officially kicked off the vaccine rollout in the country by receiving the jab himself, becoming the first non-Chinese leader to be inoculated with the Chinese vaccine. The country plans to inoculate more than 180 million of its 260 million population, in order to reach herd immunity. The president stressed that vaccinations were essential in order to end coronavirus transmissions, and to get the country's economy back on track.
In this regard, some scholars suggest that Sino-Indonesian vaccine cooperation may affect Jakarta's South China Sea (SCS) stance. Given the close vaccine cooperation with Beijing, will Jakarta compromise its commitment to a peaceful and stable SCS in accordance with international law?

Sino-Indonesian vaccine cooperation
To cope with the pandemic, the Indonesian Ministry of Foreign Affairs, in collaboration with the Ministry of State-Owned Enterprises and the Ministry of Health, intensified its vaccine hunting, exploring many possibilities of conducting cooperation with foreign companies or international consortiums to ensure its access to Covid-19 vaccines. Chinese vaccine developers were among the first to give favourable responses for establishing vaccine procurement and joint production with Indonesia.
Collaboration between a Chinese firm, Sinovac Biotech Ltd., and an Indonesian state-owned company, PT Bio Farma, has made substantial progress and created meaningful outputs. It is the only deal between the Indonesian government and foreign vaccine manufacturers that has gone beyond vaccine procurement. In Beijing, the Indonesian embassy had sought to establish contact with Sinovac since March 2020. Three months after that, Sinovac agreed to cooperate with Bio Farma in conducting clinical trials as well as vaccine manufacturing. In August 2020, a phase-three clinical trial for Sinovac's vaccine, "CoronaVac", was conducted in Bandung, involving 1,620 volunteers. Bio Farma then secured priority access to around 40 million doses of bulk vaccine from Sinovac, to be sent before March 2021. Indonesia's initial purchase of Covid-19 vaccines from Sinovac was at that time the largest order received by a Chinese vaccine developer. Bio Farma has the license to produce CoronaVac and has even been designated a production hub for the region. In March 2021, Chinese Foreign Minister Wang Yi affirmed China's readiness to "helping Indonesia to become a hub of vaccine production for Southeast Asia".
Several factors have paved the way for this close collaboration between Bio Farma and Sinovac. As stated by Djauhari Oratmangun, the Indonesian ambassador to China, the two companies "had long been in partnership" with each other. Bio Farma is also no stranger to the process of weakening or inactivating the virus to create a vaccine. Sinovac offered its vaccine at a reasonable price. In the negotiation, the Indonesian government had reportedly asked the Chinese company to ensure the transfer of technology and to guarantee that the vaccine would meet Indonesian halal standards. Moreover, Bio Farma would carry out the vaccine's mass production.
Indonesia became the first country besides China to issue emergency use authorisation of Sinovac's vaccine.
On 6 December 2020, 1.2 million doses of the Covid-19 vaccine manufactured by Sinovac landed in Jakarta. The timing could not have been more perfect; the country was entering the ninth month of the pandemic, and its daily reported positive cases had soared beyond 4,000 since the end of September. Making a public statement, President Jokowi said, "Alhamdulillah, the vaccine is available, which means that we can immediately prevent the spread of the Covid-19 outbreak." Kompas, a leading national newspaper, further praised what the president portrayed as "kabar baik" (good news) as a "victory of [Indonesia's] diplomacy". Indonesia had started to receive the vaccines while "100 other countries have not yet secured access to vaccines".

This development enabled Jokowi's government to take more concrete actions in commencing massive vaccination. Indonesia became the first country besides China to issue emergency use authorisation of Sinovac's vaccine. On 11 January 2021, the BPOM approved the emergency use of Sinovac's vaccine, since the interim results of the vaccine's phase three clinical trials in Bandung showed an efficacy rate of 65.3 - way above the standard required by the World Health Organization. Accordingly, the Majelis Ulama Indonesia (Indonesian Ulema Council, MUI) issued a fatwa reaffirming the vaccine's halal status, which had been declared four days earlier. On 13 January 2021, the Indonesian government further proceeded by conducting the inaugural Covid-19 mass vaccinations.
As seen from its Covid-19 and economic statistics, Indonesia is in an urgent situation. President Jokowi had endeavoured to expedite the mass vaccination programme since its economy continued to suffer from the pandemic. The Chinese commitment to provide Indonesians with one hundred million doses of Covid-19 vaccines enabled the latter to initiate nationwide vaccination at a relatively early time. This was great since the spread of infections was in its worst period at the beginning of 2021. On the day the president got inoculated, Indonesia reported new daily Covid-19 records with more than 11,000 new cases, 50,000 infections in the last five consecutive days, and more than 300 deaths. In that context, the country's business community enthusiastically welcomed the rollout's commencement.
On 2 March 2021, Indonesia still ranked first, third, and fifteenth in Southeast Asia, Asia, and the world respectively, where Covid-19 infections were concerned. The government authorities also announced that it had just detected the first two Covid-19 B117 variant cases in the country.
On the other hand, the mass vaccination programme continued to make progress, albeit slowly. Thanks to the continuous supply of Sinovac's vaccine, the country had seen most of its medical workers inoculated and vaccination had started for its senior citizens, merchants at public markets, clerics, teachers, journalists, athletes, public servants, government officials, military personnel and policemen. The vaccination programme was however progressing at a slow pace. After around seven weeks, only around 1% of the population had received their first vaccine shot. For the government, nevertheless, even at such a pace, the vaccination programme was regarded to be of "no mean achievement" (capaian yang baik), especially when many other countries were still struggling to ensure access to vaccines. The government's spokesperson for Covid-19 vaccination, Reisa Broto Asmoro, conveyed: "We are grateful that Indonesia is among the group of nations that have commenced [the vaccination programme]."
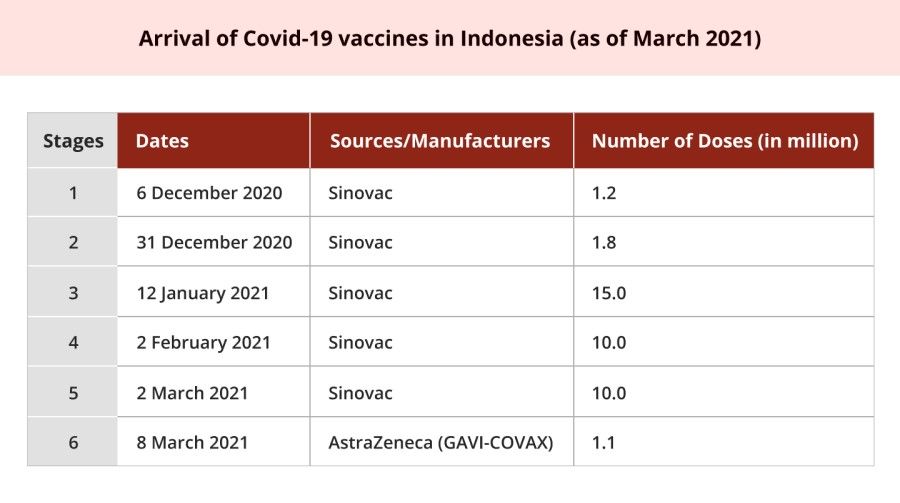
Based on the timing and the vaccine's purchase volume, Indonesia's vaccination programme has been labelled "the first large-scale use outside of China of the Sinovac Biotech Ltd. vaccine". This leads to a portrayal of the country as being heavily reliant on China in coping with the pandemic, thereby putting its independence at risk.
However, data shows that Jakarta has tried to diversify its Covid-19 vaccine sources. Sinovac's vaccines comprise about 38% of the Indonesian government's total firm orders of 329.5 million doses of vaccines. Moreover, the sum of firm orders and optional supplies from all secured sources (excluding those of Sinovac) amounts to 438 million (66% of the total). It would appear that the country is still far from being in danger of being dependent on China.
It is predicted that Bio Farma will start producing the vaccine no earlier than mid-2022.
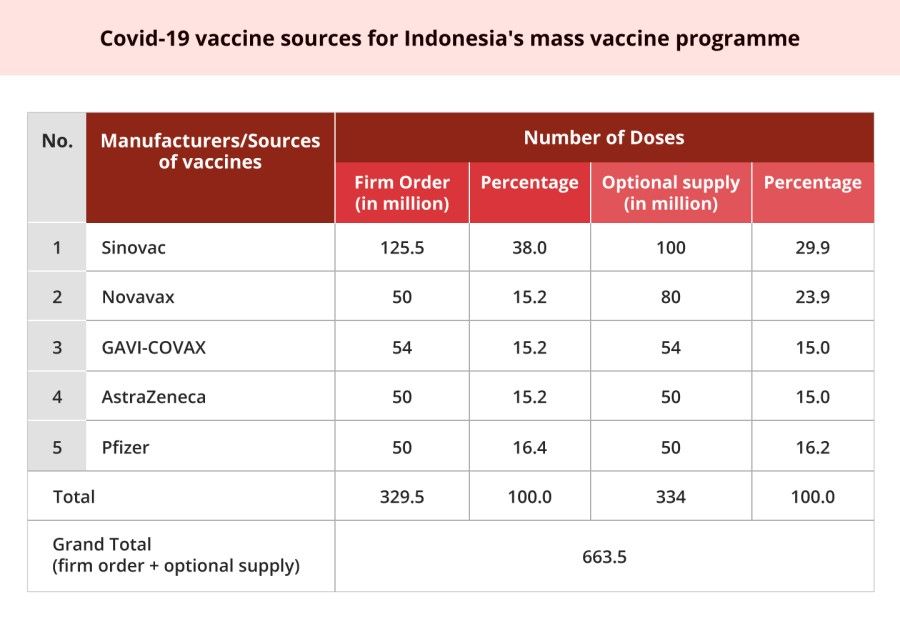
Moreover, from the perspective of the Indonesian government, procuring vaccines from China and other external sources is merely a short-term strategy to curb the pandemic. In the long run, as the Minister of Research and Technology, Bambang Brodjonegoro said, "[Indonesia] still has to have the capability of developing a vaccine domestically". In September 2020, the Indonesian government established Konsorsium Vaksin Nasional (National Vaccine Consortium), mobilising resources from the Eijkman Institute for Molecular Biology, Indonesian Institute of Sciences (LIPI), the Universitas Indonesia (UI), the Bandung Institute of Technology (ITB), Airlangga University, and Udayana University. At the time of writing, the Eijkman Institute, which has been developing a Covid-19 vaccine since April 2020, is the frontrunner in homegrown vaccine development. On 16 March 2021, the head of the institute stated that the vaccine seed would be handed over to Bio Farma at the end of the month, so that the company could start pre-clinical trials as early as April 2021 and the first phase clinical trial two months later.
It is predicted that Bio Farma will start producing the vaccine no earlier than mid-2022. The Indonesian government has allocated the use of the homegrown vaccine to the final stage of the mass vaccination programme, ensuring that the whole population will be vaccinated. In addition, the homegrown vaccine has been designated for use in the revaccination programme to boost the immune response of particular sections of the population. This strategy aims to stop Indonesia from being completely reliant on imported vaccines, including the ones from Chinese manufacturers, in dealing with the pandemic in the long term.
ASEAN states' strategy to avoid over-reliance on China
Indonesia is not the only country in the region trying to avoid over-reliance on China's vaccine. ASEAN countries are attempting to diversify the sources of vaccine supply. The table below demonstrates that the Chinese vaccine is not the only source of supply for any Southeast Asian country.
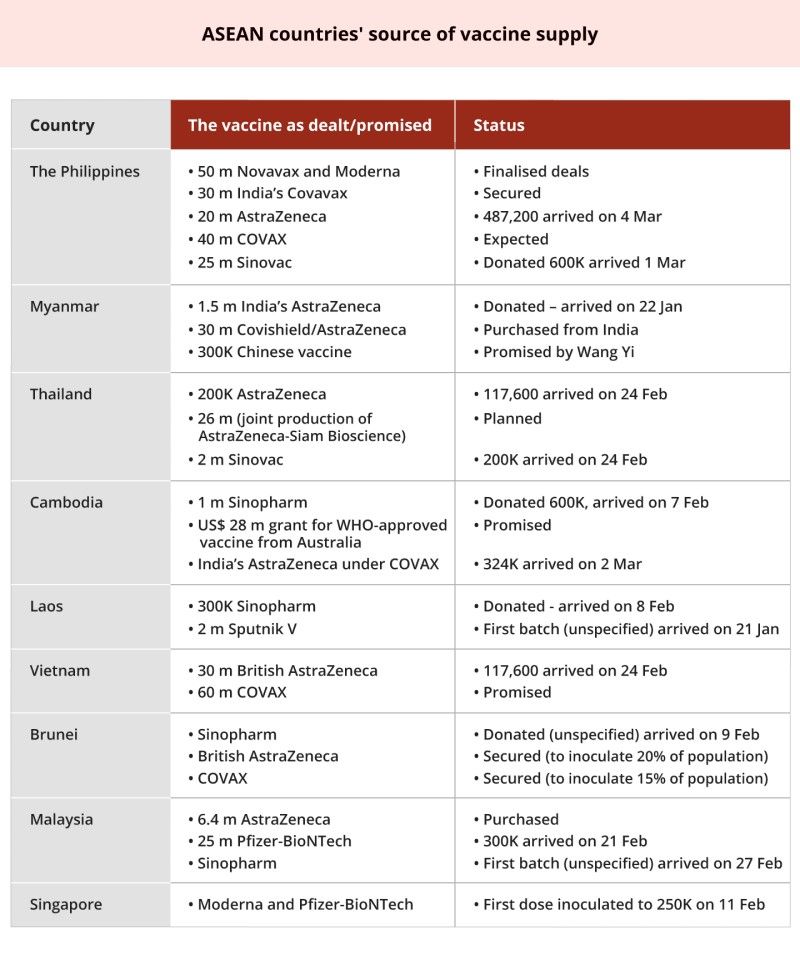
Besides avoiding dependency on China, another drive for diversification is the relatively low effectiveness of the Chinese vaccine (50.4% for Sinovac, 79% for Sinopharm and 66% for CanSino). While rich countries hoarded more reliable vaccines like that of Pfizer-BioNTech (95%), Moderna (95%), Novavax (89%) and AstraZeneca (62%-90%), China's market is more focused on developing countries. Nevertheless, India and Russia vaccine manufacturers are also aiming for developing country-markets.
Vaccine cooperation must not muzzle the country from urging China to behave lawfully in the SCS.
Jakarta's SCS diplomacy amid the pandemic
ASEAN states' diversification of vaccine supply demonstrates their reluctance to be too dependent on the Chinese vaccine, for fear of over-reliance. A deadlock at the ASEAN Foreign Minister Meetings in 2012 and 2016, for instance, should not recur. In those stalemates, Cambodia did China a favour by opposing any reference to the SCS in the agreement. The fact that the Chinese vaccine does not dominate the vaccine supply in Southeast Asian countries so far, to some extent, reduces the danger of discord among ASEAN states and enables Indonesia to lead ASEAN on the SCS issue.
Obviously, there is a link between the vaccine and the SCS issue. During the State of the Nation Address given on July 2020, Philippine President Duterte pleaded for the Covid-19 vaccine from Beijing. He suggested not confronting China in the SCS and decided to refuse a reestablishment of the US military base. During Chinese Foreign Minister Wang Yi's visit in January 2021 to the Philippines, China donated 600,000 vaccines and asked Manila to show "friendly exchanges in public, like control your megaphone diplomacy a little" on the SCS issue, according to a diplomatic source. In addition, Vietnam is uncomfortable with the idea that it has to depend on Chinese vaccine. Therefore, the country developed its homegrown Nano Covax vaccine for "national security purposes", to shut China out amid the territorial dispute in the SCS.
The SCS is an issue in which China is in a disadvantageous position legally and diplomatically. The area has already become a field of great power rivalry. In anticipation of the talks on the Code of Conduct (CoC) in the SCS, China will need cooperation from ASEAN states. On the other hand, ASEAN wants to maintain peace and stability in the SCS. Indonesia is a central figure for this purpose. The country has been hosting a workshop on Managing Potential Conflict in the SCS since the 1990s. In September 2012, Indonesia came up with the Zero Draft to advance the CoC talk.
Vaccine cooperation must not muzzle the country from urging China to behave lawfully in the SCS. In 2020, when Indonesia was intensively discussing vaccine cooperation with China, Jakarta sent two diplomatic notes to the UN secretary general (26 May and 12 June) rejecting China's SCS claim, quoting the 2016 tribunal award. For Jakarta, the 2016 tribunal award and the 1982 UNCLOS are inseparable and prerequisites for peace and stability in the SCS.
Following the incident with Chinese fishing vessels violating Indonesia's EEZ in the Natuna waters in January 2020, the Indonesian Foreign Ministry issued a statement against China's SCS claim by invoking the 2016 tribunal award. This was the first time that a third party had done so. The 2016 tribunal award which invalidates China's nine-dash line is confirmation that China's maritime rights claim and activities in the Natuna waters are unlawful. Hence, Jakarta resolutely rejected Beijing's offer for a "negotiation" in June 2020 since there were no overlapping claims.
Friendly and mutually beneficial cooperation with China needs to be conducted without compromising regional peace and stability in accordance with the law.

In early July 2020, China held a military drill near the Paracel Islands which are claimed by both Beijing and Hanoi, while Washington deployed two aircraft carriers USS Nimitz and USS Ronald Reagan to "support a free and open Indo-Pacific". Responding to the increasing tension in the SCS, Foreign Minister Retno Marsudi held a virtual bilateral meeting with her Chinese counterpart on 30 July. At the event, Indonesia urged China to comply with the 1982 UNCLOS in settling disputes.
The following month, at the 53rd anniversary of the establishment of ASEAN (8 August), Indonesia initiated the ASEAN Foreign Ministers' Joint Statement to Maintain Peace that decried "the changing geo-political dynamics" that may have "detrimental ramifications for the region". Furthermore, ASEAN Foreign Ministers also urged all parties to "resolve differences and disputes by peaceful means in accordance with international law".
On 13 January 2021, the day President Jokowi got his first shot of the Sinovac vaccine, Foreign Minister Retno Marsudi met up with the visiting Chinese Foreign Minister Wang Yi in Jakarta. Retno urged China to respect the 1982 UNCLOS and maintain stability in the SCS. She conveyed her message on behalf of ASEAN and stressed Indonesia's commitment to ASEAN centrality in a stable, peaceful, and prosperous Indo-Pacific. Indonesia has been marshalling its ASEAN counterparts in a low profile manner to advance the progress of CoC negotiations - which is scheduled to reach a conclusion in 2021. Indonesia is the most fitting actor to perform the role since it is the largest Southeast Asian state, a natural leader of ASEAN, an SCS littoral state, and not an SCS claimant.
On the margins of the 13th National People's Congress held on 7 March 2021, Wang Yi expressed his commitment to a peaceful and stable SCS. He reiterated that China would cooperate in formulating the CoC to "build consensus, enhance mutual trust, advance cooperation and maintain overall stability in the SCS". However, this commitment may not be sufficient for the region. Maintaining peace and stability in the SCS may need to find concrete form in a UNCLOS-based CoC. The boldness to urge China to respect the 1982 UNCLOS in dealing with the SCS issue is a litmus test for Indonesia and the region's independence. Friendly and mutually beneficial cooperation with China needs to be conducted without compromising regional peace and stability in accordance with the law.
Conclusion
Some scholars suggest that Sino-Indonesian vaccine cooperation may raise Jakarta's dependency on Beijing and eventually lead to Jakarta compromising on its SCS stance. While this article argues that there is a link between China's vaccine diplomacy and the SCS issue, vaccine cooperation does not in any way soften Jakarta's SCS stance. Sino-Indonesia vaccine cooperation is indeed asymmetrical, but it has not been entirely in favour of the former. Thanks to its early and large vaccine supply deal with Sinovac, Indonesia has been among the first countries to start a mass inoculation programme.
It should also be noted that Jokowi's government has so far maintained the commitment to diversify sources for Covid-19 vaccines, procuring a hundred million doses of vaccines from various non-Chinese manufacturers. In addition, the government has ensured the development of homegrown vaccines. This further suggests that Indonesia has been attempting to manage the potential risks which have emerged from its asymmetrical vaccine cooperation with China. In preventing itself from being heavily reliant on Beijing, Indonesia has so far refrained from putting its independence at risk in managing its overall bilateral relations with China.
This fact enables Jakarta to maintain its longstanding stance in the SCS. Indonesia resolutely maintains its pro-UNCLOS stance, and furthermore invoked the 2016 tribunal award that invalidates China's SCS stance in its two diplomatic notes to the UN Secretary General in 2020 - the first third party from ASEAN to do so.
The tendency to avoid over-reliance on Beijing is prevalent among ASEAN states. These countries attempt to diversify their vaccine sources. Interestingly, Vietnam is developing its homegrown vaccine for 'national security purposes'. This fact may pave the way for Jakarta's consolidation of ASEAN on the SCS issue. Amid the pandemic, Indonesia has been playing a leadership role in urging China to comply with the 1982 UNCLOS. This role is most fitting for Indonesia since it is the ASEAN central figure, the largest ASEAN nation, an SCS littoral state and a non-claimant state.
In the upcoming CoC talk, Indonesia must continue to solidify ASEAN's position in formulating a UNCLOS-based CoC in the SCS.
This article was first published as ISEAS Perspective 2021/55 "Indonesia-China Vaccine Cooperation and South China Sea Diplomacy" by Ardhitya Eduard Yeremia and Klaus Heinrich Raditio.
Related: Winning Indonesia over: US and China seek Indonesia's support in Southeast Asia | [South China Sea] Pandemic and US-Japan support reasons for Indonesia's strong stance on SCS | 'Promiscuous diplomacy': How ASEAN navigates Indo-Pacific polemics and potentials | Vaccine diplomacy: China and India push ahead to supply vaccines to developing countries

